The pharmaceutical industry is undergoing rapid transformation, with continuous advancements in drug development, technologies, and strategic collaborations. This dynamic landscape is shaped by regulatory updates, innovations in treatment, and new opportunities that redefine healthcare. In this newsletter, we cover top news stories that highlight developments, breakthroughs, and effective marketing strategies that are shaping the future of healthcare. As the pharmaceutical sector evolves, Fullintel Hub provides a trusted source of comprehensive insights, offering timely and relevant news to help you stay informed on the latest industry trends.
Top Coverage Highlights the FDA’s Approval of OTC Hearing Aid Software for Apple AirPods, Neuralink Blindsight’s ‘Breakthrough Device’ Recognition, and Eli Lilly’s Launch of Affordable Zepbound Vials
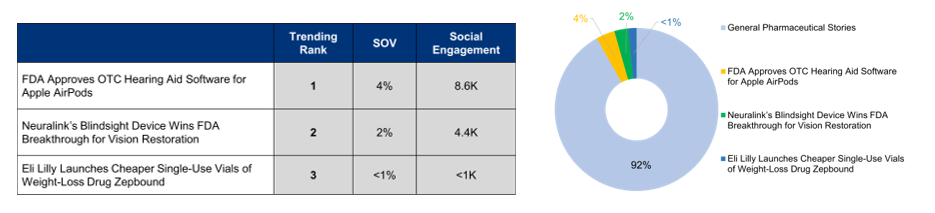
This month’s newsletter covers three healthcare innovations with potential positive societal outcomes: the FDA-approved over-the-counter (OTC) hearing aid software for Apple AirPods Pro, improving hearing care accessibility; Neuralink’s Blindsight brain implant gaining FDA breakthrough status for its progress in restoring vision for the blind; and Eli Lilly’s introduction of more affordable single-use vials of the weight-loss drug Zepbound, enhancing access to obesity treatment.
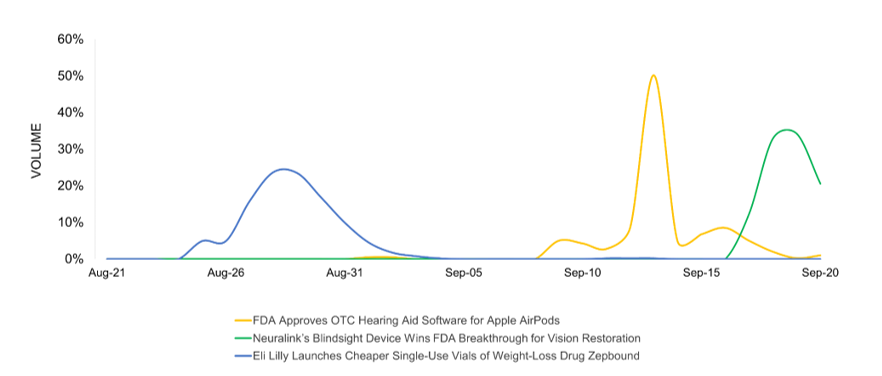
The hearing aid software for Apple AirPods Pro is the biggest news driver this month in the pharma industry. It garners the most headlines, the most volume, and the most shares making it the top-ranked story in trending coverage. Elon Musk-owned neurotechnology firm Neuralink continues to maintain its media presence with the FDA breakthrough status for Blindsight, a brain implant aimed at restoring sight for the blind. Accessibility of weight-loss drugs continues to capture media attention, with Eli Lilly’s launch of cheaper single-use vials of its popular weight-loss drug Zepbound.
A Break-Down of Recent Trending Stories:
FDA’s Approval of OTC Hearing Aid Software for AirPods Drives High Media Coverage and Engagement
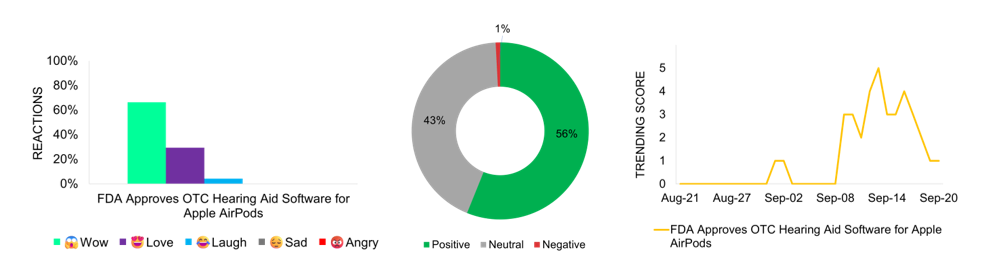
The FDA approved the first over-the-counter (OTC) hearing aid software for Apple AirPods Pro—marking a significant advancement in accessible hearing technology. This approval enables AirPods Pro 2 to function as hearing aids for individuals with mild-to-moderate hearing loss, with personalized settings available through an iPhone and iOS HealthKit. The approval aligns with the FDA’s 2022 initiative to expand access to OTC hearing aids, addressing a major public health issue affecting more than 30 million Americans. This software-based solution offers an innovative alternative to traditional hearing aids, potentially encouraging adoption among individuals hesitant to seek medical intervention. The news gains widespread media coverage and trends on September 13, the day after the approval, reflecting substantial public interest. The story generates the highest social media engagement this month, with positive Facebook reactions, as users are amazed by AirPods’ transformation into hearing aids, merging everyday technology with medical assistance. Media coverage is largely positive, praising the increased accessibility, while slight negativity stems from privacy concerns or doubts about the software’s effectiveness compared to traditional hearing aids.
.
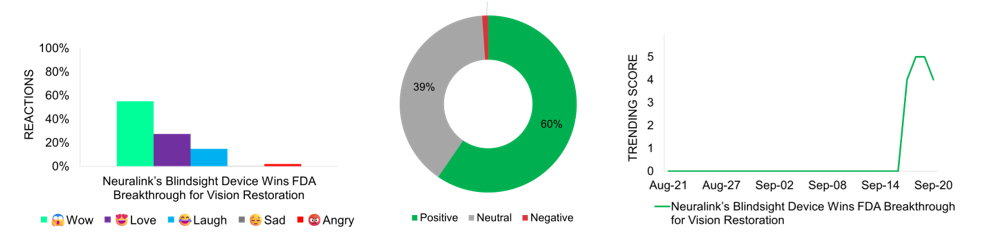
Neuralink, Elon Musk’s neurotechnology venture, once again receives favorable media attention after securing FDA breakthrough designation for its Blindsight device. This device aims to restore vision in individuals who have lost sight in both eyes and their optic nerve. The brain implant uses Neuralink’s brain-computer interface technology seeking to restore sight through direct brain stimulation. This development, along with Neuralink’s ongoing PRIME study, represents neurotechnology progress and healthcare innovation. It offers new hope to those with severe vision impairments and underscores the advancements in brain-computer interface technology. The news, which broke on September 17, quickly gains media attention and reaches peak trending status on September 18 and 19. The general reception is favorable, with most social reactions expressing awe. Media coverage is largely positive, with minimal concerns arising from ethical considerations and skepticism regarding long-term safety implications. The story garners the second-highest engagement of 4.4K and is expected to continue generating media buzz in the coming month.
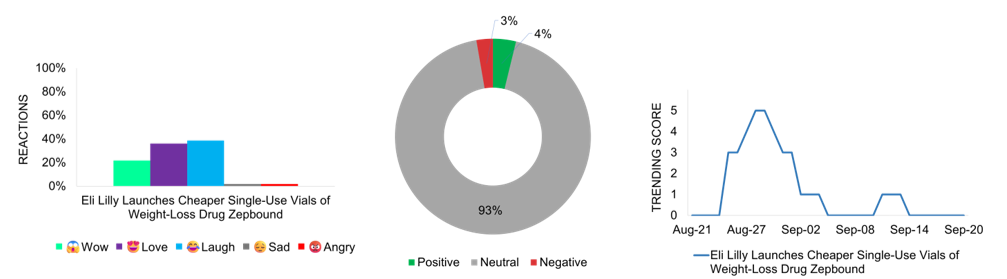
Weight-loss treatment coverage is on the rise in the pharmaceutical media space, with Eli Lilly’s launch of cheaper single-use vials for its weight-loss drug Zepbound making headlines on August 28 and 29. The brand improves access to obesity treatments with more affordable vials, at half the price of its injector pen versions. The new vials, priced at $399 for 2.5 mg and $549 for 5 mg for a four-week supply, are expected to reshape the market. Sold directly through Eli Lilly’s LillyDirect platform, this approach bypasses traditional pharmacies, simplifying the process for patients. Media coverage surrounding this news is predominantly neutral, with most reports maintaining an objective stance, reflecting the challenge of balancing increased accessibility with concerns over widespread drug use and potential misuse. The “Laugh” reactions on social media highlight skepticism that a cheaper version of an expensive drug is still priced at $399-$549 for a four-week supply, as it remains unaffordable for many.