This month, the pharmaceutical industry evolves through technological innovation, regulatory oversight, and government policy. Regulatory agencies like the FDA critically shape drug development, determining market entry and research investments. Pharmaceutical companies must navigate complex approval processes, balancing cutting-edge scientific advancements with stringent health standards. Technological breakthroughs increasingly intersect with regulatory mechanisms, creating a dynamic landscape where innovation, governance, and healthcare convergence define the sector’s future trajectory. Amid this complex ecosystem, the Fullintel Hub provides comprehensive, up-to-date insights to track these critical developments in the pharma space.
Top Pharma Stories This Month Include Trump’s Nomination of Robert F. Kennedy Jr. to Lead the HHS, FDA’s Introduction of New Rules for Clearer TV Drug Ads, and Merck’s Acquisition of Modifi Biosciences
Three key events in the healthcare space gain significant media attention this month: 1) RFK Jr.’s HHS nomination by Trump sparks debate, challenging conventional public health leadership and vaccine policy perspectives; 2) FDA’s mandate of clearer drug advertisements, requiring simplified language and more transparent risk communication in television pharmaceutical marketing; 3) Merck’s acquisition of Modifi Biosciences for $1.3B, expanding their cancer therapy portfolio and preparing for Keytruda’s patent expiration.
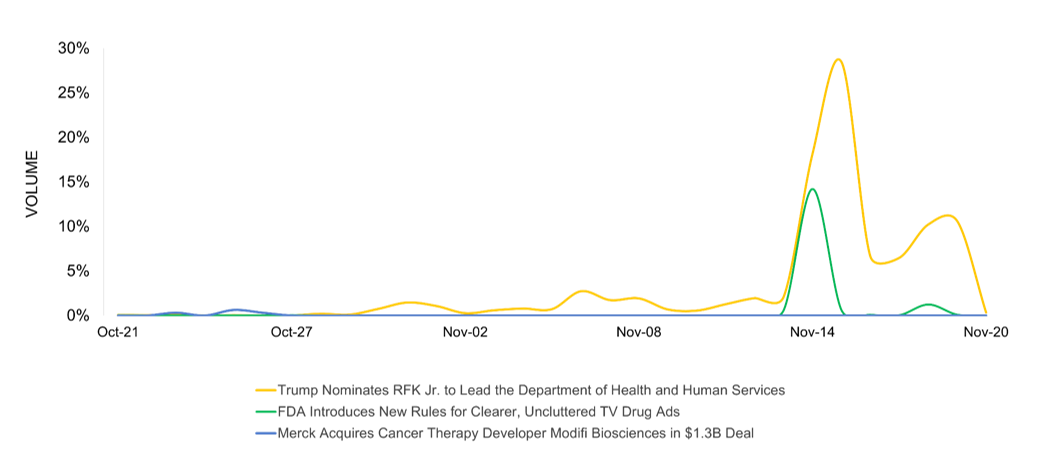
November’s pharma news is led by Trump’s controversial nomination of RFK Jr. to HHS, sparking intense debate about public health leadership and challenging conventional vaccine policy, garnering the highest social engagement. Media interest spikes followed by the FDA’s groundbreaking rules mandating clearer, more transparent drug advertisements, signaling a significant shift in pharmaceutical marketing communication and consumer information transparency. Merck’s $1.3B acquisition of Modifi Biosciences generates notable coverage, highlighting the company’s strategic expansion in cancer therapy research and preparation for Keytruda’s upcoming patent expiration.
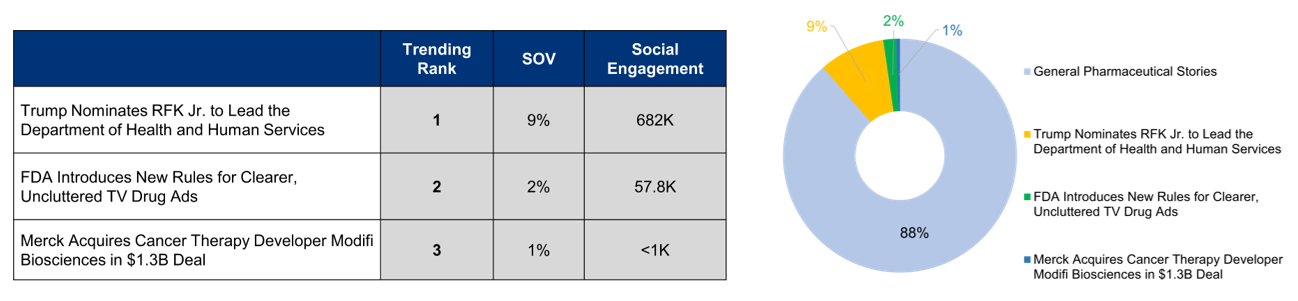
A Break-Down of Recent Trending Stories:
RFK Jr.’s Pick by Trump as Health Secretary Becomes the Most Discussed Story in the Healthcare-Political Space
President-elect Donald Trump nominates Robert F. Kennedy Jr. as Secretary of Health and Human Services, following Kennedy’s decision to suspend his independent presidential campaign and endorse Trump. The environmental lawyer and prominent vaccine skeptic’s nomination to lead the department overseeing Medicare and Medicaid programs sparks immediate debate in healthcare circles. Public health experts express serious concerns about Kennedy’s controversial health policy positions as he prepares to face Senate confirmation hearings. The nomination generates polarized reactions across social media platforms, with audiences divided between opposition and support, revealing mixed reactions. Over three-fourths of the media coverage maintains a neutral stance, apparently waiting for more details to emerge. What begins as a minor announcement quickly evolves into a major national discussion point and reaches its peak trending score of 5 on November 14th and 15th. The story maintains significant public interest and generates sustained debate across various platforms.
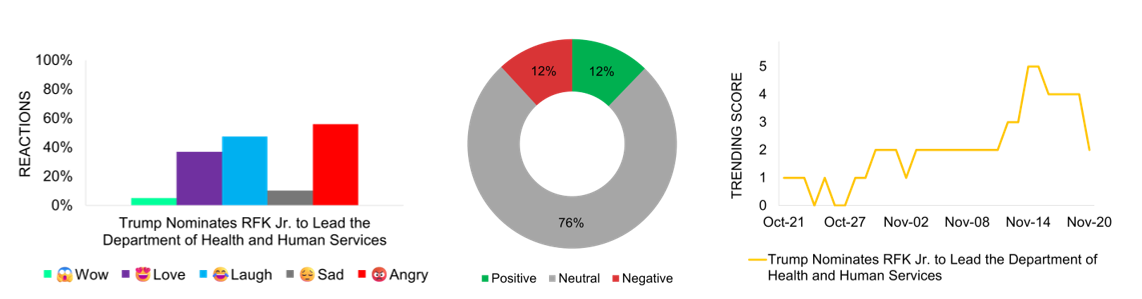
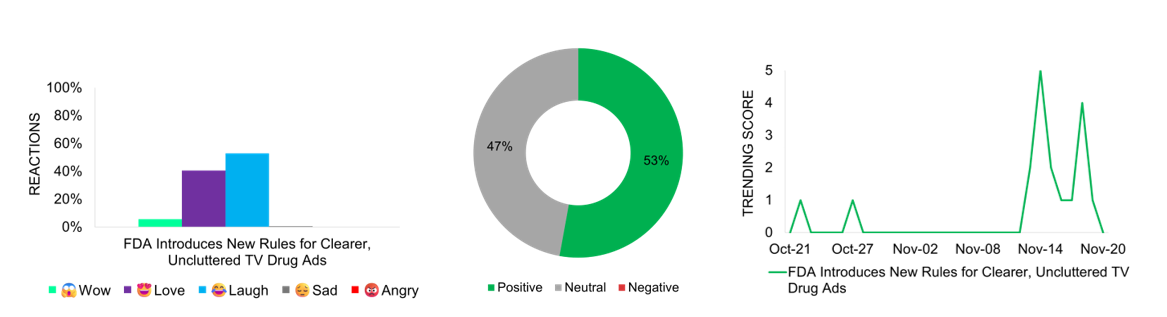
Social Audience Cheers FDA’s Crackdown on Confusing Drug Ads with New Transparency Rules, Generating Social Engagement
The FDA introduces new rules for television drug advertisements, requiring clearer language and fewer distractions when presenting medication risks and side effects. This marks a significant shift in the FDA’s stance after 15 years of development, with this regulatory change coming into effect from November 20th. The story garners significant social media engagement, with sarcasm driving social reactions awaiting proof of the rules’ effectiveness and their impact on addressing misleading advertising practices. “Love” reaction follows, suggesting a positive reception of the stricter advertising guidelines, and the dominant media perception remains favorable. The news trends mid-November, with trending scores reaching 5, likely due to the surfacing of the announcement and the approaching implementation deadline. While traditional TV remains the primary pharmaceutical advertising platform, with over $4B spent annually, the story also highlights growing concerns about unregulated pharmaceutical promotions by social media influencers. The FDA’s move represents a broader push for transparency in pharmaceutical marketing, though experts note potential loopholes in the new requirements.
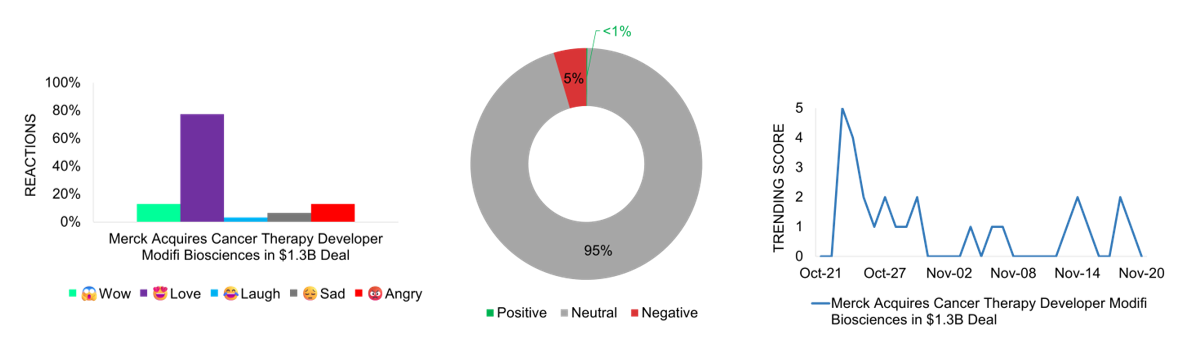
Merck makes a bold move in cancer therapy development by acquiring Modifi Biosciences in a deal worth up to $1.3B. The agreement includes a $30M upfront payment with additional milestone-based payments, focusing on treatments for difficult-to-treat brain tumors, including glioblastomas. The acquisition comes as Merck prepares for its blockbuster cancer drug Keytruda’s patent expiration by the decade’s end. Social media response shows strong public support, with Facebook users expressing primarily “Love” reactions, reflecting hope for cancer treatment advances, and “Wow” responses to the deal’s magnitude. Media coverage balances enthusiasm with appropriate caution regarding early-stage drug development. The announcement garners peak interest with a trending score of 5 on October 22nd, the announcement day, marking significant market attention. The deal represents Merck’s strategic effort to strengthen its oncology pipeline while addressing critical unmet needs in brain cancer treatment.